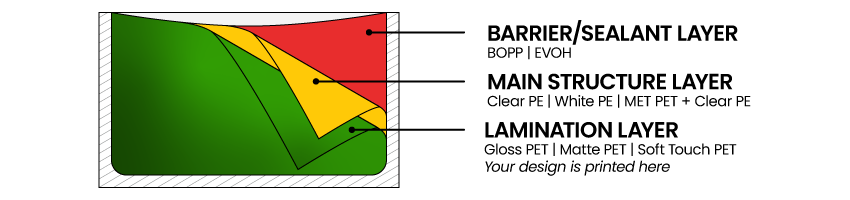
PET / METPET:
FDA 21 CFR 177.1630 compliant
PE:
FDA 21 CFR
175.105, 175.300(b)(3)(xxx), 175.320(b)3iii, 176.180, 176.210, 177.1200 , 177.1210, 177.1350, 177.1520(a)(2)(i), 177.1520(a)(3)(i), 177.1520(a)(3)(i)(a)(2) , 177.1520(a)(3)(i)(c)(1), 177.1520(b), 177.1520(c)2.1, 3.1a, 178.2010(b) , 178.3120, 178.3297(e), 178.3400, 178.3750, 178.3860, 179.45 , 181.28, 181.29, FCN 1753
Conditions of Use:
Conditions of Use B - H, as described in Table 2 of 21 CFR 176.170(c) For use in contact with nonalcoholic food. Not for use in contact with infant formula and breast milk but may be used in repeat use articles for feeding infant formula and human milk as well as packaging of powdered infant formula.

 Create a Support Ticket
Create a Support Ticket Call 1-866-899-2499
Call 1-866-899-2499 Chat with an Agent
Chat with an Agent Go to Support Center
Go to Support Center








Validate your login